At Which of the Following Temperatures Is the Average Kinetic
You have five molecules with the following speeds. A dozen eggs sat.
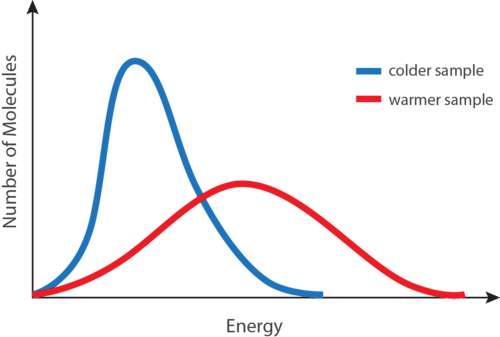
Average Kinetic Energy And Temperature Ck 12 Foundation
The right answers are.

. As the temperature is raised from 20 C to 40 C the average kinetic energy of neon atoms changes by a factor. In a 20º C room for 2 hours In 2º C refrigeration for 4 hours. The molecules have twice the average kineticenergy if the absolute temperature doubles.
A closed vessel contains a mixture of two diatomic gases A and B. The following statements are given i Average kinetic energy per molecule of A is equal to that of B. The sum of the particles kinetic energies divided by 1000.
The average kinetic energy of O 2 at a particular temperatures is 0. How is a Celsius temperature reading converted to a Kelvin temperature reading. K E 2 K E 1 T 2 T 1 40 273 20 273 313 293.
Particles at the same temperature have the same average kinetic energy so argon and nitrogen which are at 20C. Average kinetic energy is defined as the average of the kinetic energies of all the particles present in a system. The average kinetic energy of N 2 molecules in eV at the same temperature is.
Where T is the Kelvin temperature and k is Boltzmanns constant. Which of the following is a measure of the average kinetic energy of the particles in a sample of matter. Note that even though two objects can have the same temperature and therefore the same average kinetic energy they may have different internal energies.
A substance has a higher temperature when its particles have a higher average kinetic energy. Correct answer - At which of the following temperatures is the average kinetic energy of the molecules of a substance the greatest. You are sure to get a 100 have a great rest of your day.
Helium has both the smallest particles and is at the high temperature of 100C so it will be the gas with the highest speed. Which temperature scale is an absolute scale meaning temperature is directly proportional to average kinetic energy. 4The total kinetic energy of all particles in a substance.
The average kinetic energy increases. As the temperature is raised from. WHAT IS MEAN KINETIC TEMPERATURE.
Consider the following example. The average kinetic energy of neon atoms changes by a factor. Enthalpy measures the total kinetic energy of the particles in a substance.
Argon has the biggest particles and is at the low temperature of 20C so it will be the gas with the highest speed. Use the following formula for the average kinetic energy of an ideal gas per molecule. According to Kinetic Molecular Theory the average kinetic energy of molecules is a function only of temperature.
Which of the following measures the average kinetic energy of particles. Temperature is a measure of the average value of the kinetic energy of the particles in an object. 3heat flows from hot to cold and is the transfer of thermal energy.
AskedNov 5 2018in Chemistryby Aria60kpoints At which of the following temperature would the molecules of a gas have twice the average kinetic energy they have at 20C. The average kinetic energy and temperature of a system are determined by the measure of the kinetic energy of the molecules inside it. From above it is visible that kinetic energy is directly related to.
7 6 8 eV. Answer 1 of 6. The total kinetic energies of the particle plus 1000.
Examining this equation the average kinetic energy is given in Joules k B is Boltzmanns Constant 13810-23 JK and the temperature is given in Kelvins the SI unit of temperature. EqE frac32Nk_bT eq where E is the average kinetic energy of the gas T is the. Average kinetic energy Temperature in Kelvin.
The kinetic energy is proportional to the absolutetemperature. Molar mass of A is 16 times that of B and mass of gas A contained in the vessel is 2 times that of B. Which of the following best describes the motion of.
As the temperature of something increases the average speed of its particles increases. Mean Kinetic Temperature MKT is a simplified way of expressing the overall effect of temperature fluctuations during storage or transit of perishable goods. Temperature is defined to be a measure of the average translational or linear kinetic energy of the atoms or molecules of a substance.
Temperature and kinetic energy are related. 2293K 586K 586-273 o C. 20 o C 293K.
181 c b. A The higher the temperature the lower the average kinetic energy of the sample B At low temperatures intermolecular forces become important and the pressure of a gas will be lower than predicted by the ideal gas law. What happens to the average kinetic energy of the particles in a sample of matter as the temperature of the sample is increased.
T temperature in kelvin. C The smaller a gas particle the slower it will effuse D At a given. Which of the following statements is TRUE.
It is an average because individual particles in the substance are moving at different velocities. 2The average speed of the particles increased. The kinetic energies of the fastest particles multiplied by 1000.
It is determined by the equation.

Temperaturethermal Energyheat A Measure Of The Average Kinetic Energy Of The Particles In A Substance Degrees Fa Thermal Energy Internal Energy Science Lessons

Comments
Post a Comment